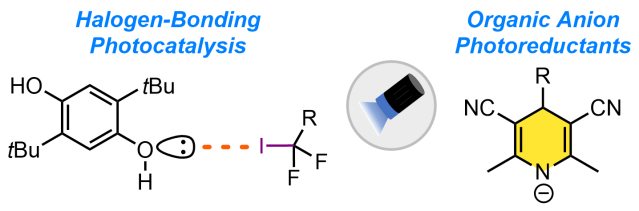
To further advance the field of radical chemistry, general strategies that serve to generate radicals directly from readily available precursors, such as alkyl halides, which circumvent the use of tin hydrides and ground state metal reductants would be of great value. In the Pitre Lab, we focus on developing organic single-electron photoreductants that can serve as a general platform for visible-light-mediated radical generation directly from unactivated precursors. Our research centers on two-distinct strategies: namely, halogen-bonding photocatalysis and organic anion photoreductants. For our halogen-bonding photocatalysis approach, we have developed Lewis base catalysts that directly engage with carbon–halide bonds via a halogen-bonding interaction, forming an electron donor-acceptor complex that can be activated by visible-light irradiation. Our second approach leverages simple 1,4-dihydropyridines anions as potent excited state photoreductants, enabling the reduction of a broad range of unactivated substrates under operationally simple and mild conditions.
Relevant Publications
Tarannum Tasnim, Calvin Ryan, Miranda L. Christensen, Christopher J. Fennell and Spencer P. Pitre*. Radical Perfluoroalkylation Enabled by a Catalytically Generated Halogen Bonding Complex and Visible Light Irradiation. Organic Letters 2022, 24, 446-450.
Prasadi G. Gallage, Mary G. McKee and Spencer P. Pitre*. 1,4-Dihydropyridine Anions as Potent Single-Electron Photoreductants. Organic Letters 2024, 26, 1975-1979.
We would like to thank the following funding agencies for supporting this work: