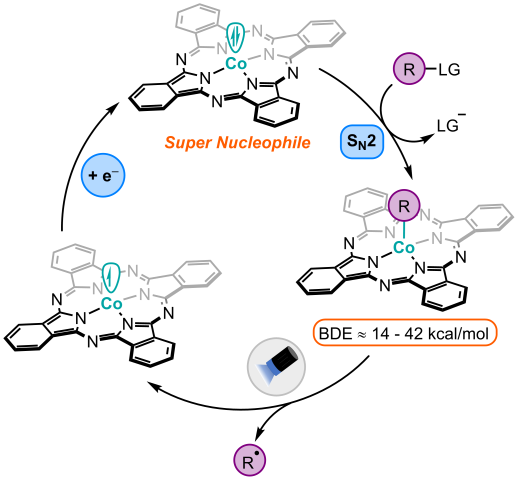
Macrocycle-ligated cobalt complexes, like Vitamin B12, are competent nucleophiles that undergo SN2 reactions with a variety of electrophiles, yielding an organometallic complex containing a Co–C σ-bond. A carbon-centered radical can then be generated through photolysis of this light-sensitive Co–C bond, which can often be done using visible-light irradiation from simple LED light sources. Our goal in the Pitre lab is to exploit the high nucleophilicity of cobalt macrocycles and their exceptional ability to generate carbon-centered radicals to develop novel transformations and to expand the scope of carbon-radical precursors that can be utilized in organic synthesis.
Relevant Publications
John Hayford G. Teye-Kau, Mayokun J. Ayodele and Spencer P. Pitre*. Vitamin B12-Photocatalyzed Cyclopropanation of Electron-Deficient Alkenes using Dischloromethane as a Methylene Source. Angewandte Chemie International Edition 2024, 63, e202316064
We would like to thank the following funding agencies for supporting this work: